
| Size | Price | Stock | Qty |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
Purity: ≥98%
Akti-1/2 (known also as AKT inhibitor VIII), a quinoxaline-based compound, is a novel, potent, selective, cell-permeable and allosteric inhibitor of Akt1/2 with potential anticancer activity. It has an IC50 of 58 nM for Akt1 and 210 nM for Akt2, respectively, and is about 36-fold more selective for Akt1 than Akt3. AKTi-1/2. Akts, PKA, PKC, and SGK lacking the pleckstrin homology (PH) domain did not exhibit any inhibition.
| Targets |
Akt1 (IC50 = 58 nM); Akt2 (IC50 = 210 nM); Akt3 (IC50 = 2119 nM)
|
|---|---|
| ln Vitro |
Akti-1/2 inhibits Akt1 and Akt2 with an IC50 of 305 nM and 2086 nM, respectively, in a cell-based IPKA (C33A) assay. Akti-1/2 causes cell apoptosis in HT29, MCF7, and A2780 cells by significantly raising caspase-3 activity. [1]
Akti-1/2 prevents insulin from controlling the expression of PEPCK, G6Pase, and FOXO1 in liver cells. [2] Akti-1/2 also strongly potentiates PAR-1-mediated platelet aggregation by blocking PKB. [3] Akti-1/2 inhibits cell growth in HCC827, NCI-H522, NCI-1651, and PC-9 cells with IC50 values of 4.7 μM, 7.25 μM, and 9.5 μM; when combined with gefitinib, Akti-1/2 results in enhanced inhibition of cell growth and apoptosis. [4] Suppressed phosphorylation of AKT and downstream GSK3β in human pluripotent stem cells, demonstrating critical role in cell survival [3] Inhibited breast cancer cell proliferation in MTT assays (specific concentrations not provided) [2] |
| ln Vivo |
Akti-1/2 (50 mg/kg, i.p.) inhibits lung Akt1 and Akt2 phosphorylation in mice at both basal and IGF-stimulated levels.AKT inhibitor VIII (50 mpk, 3 doses, ip, every 90 min) is administered to mice to achieve plasma concentrations of 1.5–2.0 μM. IGF is then administered intravenously to the animals' tail veins to promote Akt phosphorylation. Both basal and IGF-stimulated Akt1 and Akt2 phosphorylation are inhibited by IP Western in mouse lung, but Akt3 phosphorylation is unaffected.
Reduced tumor growth in breast cancer xenograft models, though dosing regimen and vehicle details were not described [2] |
| Enzyme Assay |
Briefly, a Biomek 2000 Laboratory Automation Workstation in a 96-well format is used to carry out all assays (25.5 μl at 21°C for 30 min). The addition of 10 mM MgAcetate and 5, 20, or 50 μM ATP ([γ-33P]-, 800 cpm/pmol) initiates reactions that contain 5–20 mU purified kinase and substrate protein or peptide.
Kinase activity assays used recombinant AKT proteins to confirm allosteric inhibition mechanism (methodological details not fully disclosed) [1] |
| Cell Assay |
Using the 96-hour sulforhodamine B assay (SRB), it is possible to determine how AKTi-1/2 inhibits cell growth. The sigmoidal dose-response (variable slope) equation and non-linear regression analysis are used in GraphPad Prism 6.0 to calculate the drug concentrations that inhibited 50% of cell growth (IC50) for each compound.
Western blot analysis confirmed dose-dependent reduction of p-AKT and p-GSK3β levels in treated cells [3] Colony formation assays in breast cancer cells suggested anti-proliferative effects (quantitative data not shown) [2] |
| Animal Protocol |
C57BL/6 J mice
50 mg/kg i.p. Subcutaneous xenograft models established using breast cancer cell lines; drug formulation and administration frequency were not specified [2] |
| References |
|
| Additional Infomation |
This letter describes the development of two series of potent and selective allosteric Akt kinase inhibitors that display an unprecedented level of selectivity for either Akt1, Akt2 or both Akt1/Akt2. An iterative analog library synthesis approach quickly provided a highly selective Akt1/Akt2 inhibitor that induces apoptosis in tumor cells and inhibits Akt phosphorylation in vivo.[1]
Purpose: Previous studies have reported that the Curcuma wenyujin Y.H. Chen et C. Ling extract, which has a high furanodiene content, showed anti-cancer effects in breast cancer cells in vitro. The present study was designed to evaluate the in vitro and in vivo anti-cancer activity of furanodiene. Methods: The in vitro effects of furanodiene were examined on two human breast cancer cell lines, MCF-7 and MDA-MB-231 cells. Assays of proliferation, LDH release, mitochondrial membrane potential (ΔΨm), cell cycle distribution, apoptosis and relevant signaling pathways were performed. The in vivo effect was determined with MCF7 tumor xenograft model in nude mice. Results: Furanodiene significantly inhibited the proliferation and increased the LDH release in both cell lines in a dose-dependent manner. ΔΨm depolarization, chromatin condensation, and DNA fragmentation were also observed after furanodiene treatment. Furanodiene dose-dependently induced cell cycle arrest at the G0/G1 phase. The protein expressions of p-cyclin D1, total cyclin D1, p-CDK2, total CDK2, p-Rb, total Rb, Bcl-xL, and Akt were significantly inhibited by furanodiene, whereas the protein expressions of Bad and Bax, and the proteolytic cleavage of caspase-9, caspase-7, and poly-ADP-ribose polymerase (PARP) were dramatically increased. Furthermore, the z-VAD-fmk markedly reversed the furanodiene-induced cell cytotoxicity, the proteolytic cleavage of caspase-9, and DNA fragmentation but did not affect the proteolytic cleavage of PARP, whereas the Akt inhibitor VIII increased the furanodiene-induced cytotoxicity and PARP cleavage. In addition, furanodiene dose-dependently suppressed the tumor growth in vivo, achieving 32% and 54% inhibition rates after intraperitoneal injection of 15 mg/kg and 30 mg/kg, respectively. Conclusions: Taken together, we concluded that furanodiene suppresses breast cancer cell growth both in vitro and in vivo and could be a new lead compound for breast cancer chemotherapy.[2] Human embryonic and induced pluripotent stem cells are self-renewing pluripotent stem cells (PSC) that can differentiate into a wide range of specialized cells. Basic fibroblast growth factor is essential for PSC survival, stemness and self-renewal. PI3K/AKT pathway regulates cell viability and apoptosis in many cell types. Although it has been demonstrated that PI3K/AKT activation by bFGF is relevant for PSC stemness maintenance its role on PSC survival remains elusive. In this study we explored the molecular mechanisms involved in the regulation of PSC survival by AKT. We found that inhibition of AKT with three non-structurally related inhibitors (GSK690693, AKT inhibitor VIII and AKT inhibitor IV) decreased cell viability and induced apoptosis. We observed a rapid increase in phosphatidylserine translocation and in the extent of DNA fragmentation after inhibitors addition. Moreover, abrogation of AKT activity led to Caspase-9, Caspase-3, and PARP cleavage. Importantly, we demonstrated by pharmacological inhibition and siRNA knockdown that GSK3β signaling is responsible, at least in part, of the apoptosis triggered by AKT inhibition. Moreover, GSK3β inhibition decreases basal apoptosis rate and promotes PSC proliferation. In conclusion, we demonstrated that AKT activation prevents apoptosis, partly through inhibition of GSK3β, and thus results relevant for PSC survival.[3] 3-[1-[[4-(7-phenyl-3H-imidazo[4,5-g]quinoxalin-6-yl)phenyl]methyl]-4-piperidinyl]-1H-benzimidazol-2-one is a member of piperidines. |
| Molecular Formula |
C34H29N7O
|
|---|---|
| Molecular Weight |
551.6404
|
| Exact Mass |
551.243
|
| Elemental Analysis |
C, 74.03; H, 5.30; N, 17.77; O, 2.90
|
| CAS # |
612847-09-3
|
| Related CAS # |
PF-AKT400;1004990-28-6
|
| PubChem CID |
135398501
|
| Appearance |
Light yellow to yellow solid powder
|
| Density |
1.4±0.1 g/cm3
|
| Melting Point |
242-245ºC (dec.)
|
| Index of Refraction |
1.734
|
| LogP |
5.1
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
5
|
| Rotatable Bond Count |
5
|
| Heavy Atom Count |
42
|
| Complexity |
1270
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=C1N([H])C2=C([H])C([H])=C([H])C([H])=C2N1C1([H])C([H])([H])C([H])([H])N(C([H])([H])C2C([H])=C([H])C(C3=C(C4C([H])=C([H])C([H])=C([H])C=4[H])N=C4C([H])=C5C(C([H])=C4N3[H])=NC([H])=N5)=C([H])C=2[H])C([H])([H])C1([H])[H]
|
| InChi Key |
BIWGYFZAEWGBAL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C34H29N7O/c42-34-39-26-8-4-5-9-31(26)41(34)25-14-16-40(17-15-25)20-22-10-12-24(13-11-22)33-32(23-6-2-1-3-7-23)37-29-18-27-28(36-21-35-27)19-30(29)38-33/h1-13,18-19,21,25H,14-17,20H2,(H,35,36)(H,39,42)
|
| Chemical Name |
3-[1-[[4-(7-phenyl-3H-imidazo[4,5-g]quinoxalin-6-yl)phenyl]methyl]piperidin-4-yl]-1H-benzimidazol-2-one
|
| Synonyms |
Sigma-A6730; AKT inhibitor VIII; AKT inhibitor-8; Akt inhibitor VIII; 612847-09-3; Akti-1/2; YX4CPQ6V6X; AKT-inhibitor-VIII; AKT-inhibitor-8; Akt-I 1,2; Akti-1/2
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: ~22 mg/mL (~39.9 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2 mg/mL (3.63 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2 mg/mL (3.63 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8128 mL | 9.0639 mL | 18.1278 mL | |
| 5 mM | 0.3626 mL | 1.8128 mL | 3.6256 mL | |
| 10 mM | 0.1813 mL | 0.9064 mL | 1.8128 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00897663 | Completed | Genetic: microarray analysis Genetic: gene expression analysis |
Brain and Central Nervous System Tumors |
Alliance for Clinical Trials in Oncology |
November 2006 | Phase 3 |
| NCT00671970 | Completed | Drug: Bevacizumab and Erlotinib |
Glioblastoma Gliosarcoma |
Duke University | February 2007 | Phase 2 |
|
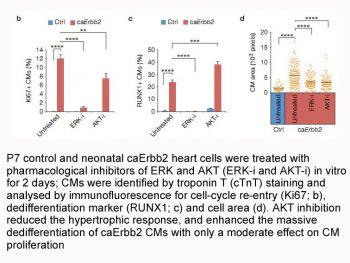 |