
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
Purity: ≥98%
Tideglusib (formerly known as NP031112, NP12) is a novel, potent, irreversible, non-ATP-competitive and chemical inhibitor of GSK-3β (glycogen synthase kinase-3β) with potential neuroprotective effects and may be used as an anti-AD (Alzheimer disease) agent. Tideglusib is presently undergoing phase II clinical trials for Alzheimer's disease and progressive supranuclear palsy; it inhibits GSK-3 with an IC50 of 60 nM in cell-free assay. The lack of recovery in enzyme activity after unbound Tideglusib was removed from the reaction medium and the fact that its dissociation rate constant is not significantly different from zero are evidence that Tideglusib inhibits GSK-3 irreversibly. Additionally, tideglusib was unable to inhibit a number of kinases with an active site Cys homologous to Cys-199, indicating that its inhibition of GSK-3 is the result of a specific mechanism and not nonspecific reactivity.
| Targets |
GSK-3β (IC50 = 5 nM); GSK-3β (IC50 = 60 nM)
|
|---|---|
| ln Vitro |
Tideglusib (NP031112) treatment completely eliminates the induction of TNF- and COX-2 expression after glutamate treatment in both astrocyte and microglial cultures. Because the 24-hour exposure of astrocyte and microglial cells to this TDZD has no effect on cell viability, these effects of NP031112 are not brought on by a reduction in cell viability[2].
|
| ln Vivo |
Tideglusib (NP031112) (50 mg/kg) injection into the rat hippocampus significantly reduces kainic acid-induced inflammation as measured by edema formation using T2-weighted magnetic resonance imaging and glial activation and has a neuroprotective effect in the damaged areas of the hippocampus[2].
Coinjection of Tideglusib (NP031112), a more potent thiadiazolidinone derivative, into the rat hippocampus dramatically reduces kainic acid-induced inflammation, as measured by edema formation using T2-weighted magnetic resonance imaging and glial activation and has a neuroprotective effect in the damaged areas of the hippocampus. Last, NP031112-induced neuroprotection, both in vitro and in vivo, was substantially attenuated by cotreatment with GW9662 (2-chloro-5-nitrobenzanilide), a known antagonist of the nuclear receptor peroxisome proliferator-activated receptor gamma, suggesting that the effects of NP031112 can be mediated through activation of this receptor. As such, these findings identify NP031112 as a potential therapeutic agent for the treatment of neurodegenerative disorders.[2] |
| Enzyme Assay |
[35S]Tideglusib (207 Bq/nmol) at 55 μM is incubated with 5 μM GSK-3β for 1 h at 25 °C in 315 μL of 50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl and 0.1 mM EGTA. The incubation is extended for another 30 min after having added 35 μL of the same buffer with or without 100 mM DTE. Finally, a third 40-μL aliquot of each original sample is mixed with 10 μL of denaturing electrophoresis sample buffer without reducing agents, and 35 μL of this mixture is loaded onto a 10% polyacrylamide gel and subjected to SDS-PAGE, followed by fluorography of the dried gel. Finally, a third 40-L aliquot of each original sample is combined with 10 L of denaturing electrophoresis sample buffer without reducing agents, and 35 L of this mixture is loaded onto a 10% polyacrylamide gel and subjected to SDS-PAGE followed by fluorography of the dried gel.[1]
Evaluation of Inhibition on Kinase Panel[1] The inhibitory activities of Tideglusib (NP031112) and hypothemycin on a panel of selected kinases were evaluated in the Invitrogen European Screening Center. Compounds were tested in duplicate at a single concentration of 10 μm on a group of selected kinases, the enzymatic activity of which was measured using the Z′-LYTETM technology at ATP and peptide concentrations around their Km values, except for MEK1, MEK2, p38α, and JNK1, for which ATP was at 100 μm. In a few cases where it was not possible to monitor the activity of the kinases (MEK3, NIK, TAK1-TAB1, MNK2, NLK, and ZAK) the ability of the compounds to displace the binding of fluorescent analogues of known ATP-competitive inhibitors was measured using the time-resolved FRET-based LanthaScreenTM technology. Details on the nature of the kinases tested and the average of the results obtained in each case are presented in supplemental Table 1. |
| Cell Assay |
Rat primary astrocytes, microglia, and neurons were harvested and cultured as described previously (Luna-Medina et al., 2005). The purity of the cultures was >95%, as determined by immunofluorescence analysis using anti-cd11b (OX-42) to detect microglial cells, glial fibrillary acidic protein (GFAP) to identify astrocytes, and anti-microtubule-associated protein 2 (MAP2) to identify neurons. Tideglusib (NP031112) (2.5 μm) was added to the culture medium of astrocytes and microglia 1 h before exposure to glutamate (500 μm), cells were incubated for 24 h before tissue culture medium was collected, and the cells were evaluated for tumor necrosis factor-α (TNF-α) and cyclooxygenase type 2 (COX-2) expression. For transient transfection experiments, primary cultures of astrocytes were transfected with the reporter plasmid pPPRE-tk-luc, containing three PPARγ consensus binding sites upstream of a minimal promoter using Transfast according to the manufacturer's guidelines. Typically, cells received 0.2 μg of luciferase reporter plasmid and were harvested 24 h after treatment with different concentrations of Tideglusib (NP031112) for determination of luciferase and β-galactosidase (to determine transfection efficiency) activities. Each transient transfection experiment was repeated at least three times in triplicate.[1]
Measurement of apoptosis. To calculate the extent of apoptotic cell death, cortical neuronal cultures were treated or not with Tideglusib (NP031112) and incubated with glutamate (100 μm), and phosphatydilserine exposure on the surface of apoptotic cells was detected by confocal microscopy after staining with Annexin V-FITC. Neuronal cell death was assessed by counting the percentage of Annexin-V-positive cells in four independent high-magnification (200×) fields per culture, as described above.[1] |
| Animal Protocol |
Rats; In this study, adult male Wistar rats (8–12 weeks old) are used. Rats (n≥5) are put into a stereotaxic machine. The hippocampus is given an injection of KA (1 μg in 2.5 μL l PBS) alone or in conjunction with Tideglusib (2 ng in 2.5 μL PBS). Animals in the control group are given vehicle injections and are the same age.
KA administration.[2] Adult male Wistar rats (8–12 weeks old) were used in this study. Adequate measures were taken to minimize pain or discomfort of animals. Experiments were performed in accordance with the European Communities Council, directive 86/609/EEC. Rats (n ≥ 5 per group) were anesthetized by intraperitoneal injection of ketamine (60 mg/kg) and Domtor (5 μg/kg) and placed into a stereotaxic apparatus. KA (1 μg in 2.5 μl PBS) alone or in combination with Tideglusib (NP031112) (2 ng in 2.5 μl PBS) was injected into the hippocampus [coordinates from bregma: posterior, −3.0 mm; lateral, −2.0 mm; depth, 3.5 mm; according to the atlas of Paxinos and Watson (1998)]. Control animals of the same age were injected with vehicle. Two groups of animals also received 0.7 μg of the PPARγ antagonist GW9662 (2-chloro-5-nitrobenzanilide), either alone or in combination with KA. Each injection was performed for >2.5 min using a micropump. The amounts of NP031112 and GW9662 used were calculated based on the in vitro results to reach active concentrations within the hippocampus. Lithium chloride (LiCl), a potent inhibitor of GSK-3β activity, was administered (40 mg/kg/d) by intraperitoneal injection to a further two groups of animals, either alone or in combination with KA. The rats were then housed individually to recover.[2] Seizures were induced by intraperitoneal administration of rats with KA (10 mg/kg) in PBS. Control animals received saline only. Behavioral analysis was monitored for a period of 3 h by trained observers blind to the treatment of the rats. The convulsive behavior was classified according to Racine (1972) and Sperk et al. (1985) as follows: stage 0, no changes; stage 0.5, wet dog shakes (WDS); stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimbs clonus; stage 4, rearing; stage 5, rearing and falling; stage 6, death. Status epilepticus (SE) was defined as continuous behavioral seizure activity (stage 5) for ≥5 min. The number of WDS before SE was also examined. In trials using Tideglusib (NP031112), the TDZD was administered intragastrically (50 mg/kg) 1 h before KA injection. |
| References |
|
| Additional Infomation |
Tideglusib is a member of the class of thiadiazolidines that is 1,2,4-thiadiazolidine-3,5-dione which is substituted by a naphthalen-1-yl group at position 2 and by a benzyl group at position 4. It is a non-ATP competitive inhibitor of glycogen synthase kinase 3beta (GSK3beta) and has neuroprotective effects. Currently under clinical investigation for the treatment of Alzheimer's disease and progressive supranuclear palsy. It has a role as an EC 2.7.11.26 (tau-protein kinase) inhibitor, a neuroprotective agent, an anti-inflammatory agent and an apoptosis inducer. It is a member of naphthalenes, a member of benzenes and a thiadiazolidine.
Tideglusib is under the investigation for the development of treatments for Alzheimer's disease and for progressive supranuclear palsy. It is reported to be a potent anti-inflammatory and neuroprotective that is a non-ATP competitive inhibitor of glycogen synthase kinase 3 (GSK-3). Tideglusib is being developed by the Spanish pharmaceutic company Zeltia group and its current status is withdrawn for the treatment of Alzheimer's disease as of 2012. Drug Indication Tideglusib was initially formulated for the treatment of Alzheimer and progressive supranuclear palsy. The raising interest for the use of tideglusib comes from the significant upregulation of GSK-3 in the brain in patients with Alzheimer disease. Its function as a degradant of β-catenin, was also important, as it prevents the transcription of cell survival genes. All these factors have directed current research towards this kinase as a potential target. Alzheimer disease is the most prevalent form of dementia. The most accepted hypothesis to explain this disease is related to the presence of amyloid β, which triggers a cascade that will alter the Tau protein and provoke synaptic dysfunction and neuronal death. GSK-3 importance in the tissue repair pathway has also pointed out a novel application for tideglusib. Thus, it is also under the research for the natural repair treatment of deep caries lesions. Mechanism of Action GSK-3 is a proline/serine protein kinase that is ubiquitously expressed and involved in many cellular signaling pathways. From all its diverse functions, it plays a key role in Alzheimer's disease. This role is related to its link with β-amyloid and tau pathology. It has been suggested that aberrant Wnt or insulin signaling results in increased GSK-3 function. This kinase acts on γ-secretase producing the hyperphosphorylation of tau, the formation of neurofibrillary tangles and senile plaques. Tideglusib inhibits GSK-3 irreversibly by presenting a non-competitive inhibition pattern with respect to ATP. The binding of tideglusib seems to directly relate to the motif containing Cys199. Pharmacodynamics It is reported that tideglusib administration inhibits the activation of astrocytes and microglial cells, thus it presented a neuroprotective effect. It is known as well that the inactivation of GSK-3 protects against excitotoxicity. In pre-clinical trials, there have been reports of decrease Tau hyperphosphorylation, lower brain amyloid plaque load, learning and memory enhancement, prevention of neuronal loss and significant increases of the insulin growth factor 1 which is a potent neurotrophic peptide with therapeutic value.The reports in clinical trials have shown a trend in cognition increase of Alzheimer patients treated for 24 weeks. |
| Molecular Formula |
C19H14N2O2S
|
|---|---|
| Molecular Weight |
334.3917
|
| Exact Mass |
334.077
|
| Elemental Analysis |
C, 68.25; H, 4.22; N, 8.38; O, 9.57; S, 9.59
|
| CAS # |
865854-05-3
|
| Related CAS # |
865854-05-3
|
| PubChem CID |
11313622
|
| Appearance |
White to off-white solid powder
|
| Density |
1.4±0.1 g/cm3
|
| Boiling Point |
511.3±43.0 °C at 760 mmHg
|
| Melting Point |
148-150ºC
|
| Flash Point |
263.0±28.2 °C
|
| Vapour Pressure |
0.0±1.3 mmHg at 25°C
|
| Index of Refraction |
1.735
|
| LogP |
3.28
|
| Hydrogen Bond Donor Count |
0
|
| Hydrogen Bond Acceptor Count |
3
|
| Rotatable Bond Count |
3
|
| Heavy Atom Count |
24
|
| Complexity |
492
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=C1N(CC2C=CC=CC=2)C(=O)N(C2C3C(=CC=CC=3)C=CC=2)S1
|
| InChi Key |
PMJIHLSCWIDGMD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H14N2O2S/c22-18-20(13-14-7-2-1-3-8-14)19(23)24-21(18)17-12-6-10-15-9-4-5-11-16(15)17/h1-12H,13H2
|
| Chemical Name |
4-benzyl-2-naphthalen-1-yl-1,2,4-thiadiazolidine-3,5-dione
|
| Synonyms |
Tideglusib; NP031112, NP-12; NP-12; NP031112; Tideglusib [INN]; 4-Benzyl-2-(naphthalen-1-yl)-1,2,4-thiadiazolidine-3,5-dione; NP-031112; tideglusibum; NP031112; NP 031112; NP-031112
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: ~66 mg/mL (~197.4 mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.48 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.48 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. View More
Solubility in Formulation 3: 4% DMSO+corn oil: 2.5mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9905 mL | 14.9526 mL | 29.9052 mL | |
| 5 mM | 0.5981 mL | 2.9905 mL | 5.9810 mL | |
| 10 mM | 0.2991 mL | 1.4953 mL | 2.9905 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05004129 | Recruiting | Drug: Tideglusib | Congenital Myotonic Dystrophy | AMO Pharma Limited | August 23, 2021 | Phase 2 Phase 3 |
| NCT05105958 | Not yet recruiting | Drug: Tideglusib | Amyotrophic Lateral Sclerosis | University Hospital, Geneva | December 1, 2025 | Phase 2 |
| NCT02858908 | Completed | Drug: Tideglusib | Myotonic Dystrophy 1 | AMO Pharma Limited | July 20, 2016 | Phase 2 |
| NCT01350362 | Completed | Drug: tideglusib Drug: Placebo |
Alzheimer's Disease | Noscira SA | April 2011 | Phase 2 |
| NCT00948259 | Completed | Drug: NP031112 Drug: Placebo |
Alzheimer´s Disease | Noscira SA | December 2008 | Phase 1 Phase 2 |
Inhibition of PI3K pathway signaling in cells. KPL-4 cells were treated with the indicated concentrations of CH5132799 for 2 hours. Clin Cancer Res, 2011, 17(10), 3272-3281. |
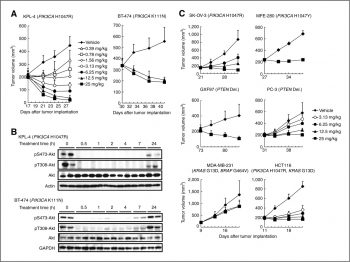 Antitumor activity in mouse xenograft models of cell lines harboring genetic alterations, including PIK3CA mutations |
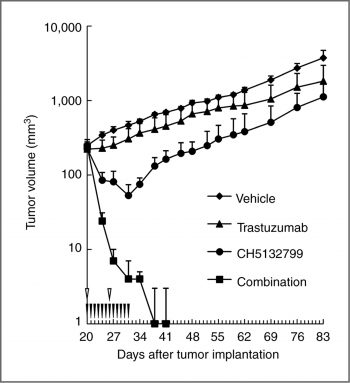 Antitumor activity in combination with trastuzumab in the trastuzumab-insensitive model. |