| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: ≥98%
| Targets |
RON (IC50 = 9 nM)
|
|---|---|
| ln Vitro |
SYN1143 (Compound I) (10-1000 nM; 1 h) suppresses HT-29 and BxPC3 cells' functional activity and c-Met-mediated signaling[1].
SYN1143 (10-1000 nM; 1 h) suppresses NIH3T3 RON and BxPC3 cells' RON-mediated signaling and functional activity[1]. SYN1143 (0.3-30 μM; 2 h or 3 d) suppresses c-Met signaling and cell proliferation in MC3T3-E1 and C3H10T1/2 cells[2]. SYN1143 (0.3-2 μM; 4-12 d) increases precursor cell osteogenic differentiation [2]. Compound I/SYN1143 inhibits the kinase activity of RON and c-Met. The structure of compound I is shown in Fig. 1A. In vitro kinase assays showed that compound I is a potent inhibitor of human RON and c-Met with IC50s of 9 and 4 nmol/L, respectively. To determine its selectivity profile, compound I was tested against a panel of tyrosine and serine/threonine kinases. Compound I had weak inhibitory activity on Lck, Tie2, Src, and BTK with IC50s ranging from 160 to 710 nmol/L (Fig. 1B) and had IC50s of >1 μmol/L on all other kinases tested (>20 kinases; Fig. 1B; data not shown). These data suggest that compound I is a selective inhibitor of RON and c-Met.[1] Compound I/SYN1143 inhibits c-Met–mediated signaling and functional activity. To confirm its activity on c-Met, compound I was evaluated for its ability to inhibit HGF-mediated c-Met phosphorylation and downstream signaling. HT-29 and BxPC3 cells were used throughout these studies because they both express c-Met and RON (Fig. 4A). In both cell lines, exogenous HGF stimulated c-Met phosphorylation and downstream signaling as evidenced by the phosphorylation of Gab1, ERK1/2, and AKT (Fig. 2A). Compound I inhibited HGF-mediated c-Met phosphorylation and downstream signaling in a dose-dependent manner. Furthermore, compound I inhibited c-Met phosphorylation with similar potency in both cell lines (Fig. 2A).[1] Compound I/SYN1143 inhibits RON-mediated signaling and functional activity. Because compound I also inhibits RON kinase activity, we evaluated its ability to block MSP-mediated signaling. Because none of the commercially available antibodies to phospho-RON recognized the appropriate molecular weight band by Western blotting (data not shown), we performed immunoprecipitation to test the effect of compound I on MSP-induced RON phosphorylation in NIH3T3 RON or BxPC3 cells. Phospho-RON was immunoprecipitated from the lysates of cells treated with MSP and/or compound I using the anti-phosphotyrosine 4G10 antibody. RON was then detected by Western blotting using an antibody against the COOH terminus of the receptor. We found that compound I inhibited RON phosphorylation in a dose-dependent manner in both cell lines (Fig. 3A, and B, top). We then tested the effect of MSP and/or compound I on downstream signaling, including the phosphorylation of Gab1, ERK1/2, and AKT, in NIH3T3 RON cells or in BxPC3. Treatment with exogenous MSP resulted in robust phosphorylation of Gab1, AKT, and ERK1/2 in NIH3T3 RON cells (Fig. 3A), whereas MSP treatment resulted in weaker phosphorylation of Gab1 and had little effect on AKT and ERK1/2 phosphorylation in BxPC3 cells (Fig. 3B). Because the only known receptor for MSP is RON, it is reasonable to conclude that the activation of Gab1, AKT, and ERK1/2 is mediated by RON. Compound I inhibited MSP-induced phosphorylation of Gab1, ERK1/2, and AKT in a dose-dependent manner in NIH3T3 RON cells (Fig. 3A) and MSP-mediated phosphorylation of Gab1 in BxPC3 cells (Fig. 3B). Compared with HGF/c-Met signaling (Fig. 2A), MSP/RON signaling is weak in BxPC3 cells and has a minor effect on ERK1/2 and AKT phosphorylation (Fig. 3B).[1] c-Met-specific small molecule kinase inhibitors SYN1143 and SGX523 inhibit c-Met signaling and cell proliferation in vitro.[2] c-Met chemical inhibitors promote mineralization of precursor cells[2] We next investigated the effect of c-Met inhibitors on osteogenic differentiation of precursor cells. MC3T3-E1 cells were treated with osteogenic media in the presence or absence of c-Met inhibitors at various concentrations 0.3–2 μM). The effects of c-Met inhibitors on osteoblast differentiation were determined by analyzing the activity of ALP, (an early phase marker) in MC3T3-E1 cells. Interestingly, c-Met inhibitor SYN1143 significantly increased ALP activity at concentration of 1 μM. The activity of ALP induced by SYN1143 was comparable to that induced by BMP2 (Fig. 2A). Extracellular matrix mineralization is the most important process during bone formation. Under the same conditions, c-Met inhibitor SYN1143 dramatically increased mineralization based on alizarin red staining (AR-S) (Figs. 2A, 2B). The ability of c-Met inhibitor SGX523 in enhancing osteogenic differentiation was also found at low concentrations (Figs. 2A, 2B). Consistent with the above findings, c-Met inhibitors also increased the expression levels of Col1a1, ALP, and BSP genes encoding the most important contents of bone matrix proteins during bone formation (Alford et al., 2015) (Figs. 2C-2E). Similar effects were observed in C3H10T1/2 cells, although the ability of c-Met inhibitors in enhancing osteogenic differentiation in C3H10T1/2 cells was lower than that in MC3T3-E1 cells (Figs. 2F, 2G). These results suggest that c-Met inhibitors SYN1143 and SGX523 might potentiate osteogenic differentiation of precursor cells. SYN1143 or SGX523-mediated c-Met inhibition increases Runx2 via pathway independent of Erk-Smad[2] It has been reported that HGF can inhibit BMP2 signaling pathway by preventing active phosphorylation of Smads in hMSCs and C2C12 cells (Standal et al., 2007). To understand the mechanism by which c-Met inhibitors regulate osteoblast differentiation, we first checked the effect of c-Met inhibitors on BMP2-Smad signaling activity. Treatment with SYN1143 or SGX523 did not significantly affect the levels of active forms of Smads phosphorylation (phosphorylation on Ser463/465 of Smad1 and Ser463/465 of Smad5) under both non-osteogenic and osteogenic inducing conditions (Figs. 3A, 3B). It has been reported that inhibiting phosphorylation of Smad1 function including phosphorylation of Ser206 at the linker region of Smad1 can be resulted from Erk1/2 activation (Kretzschmar et al., 1997, Sapkota et al., 2007) and HGF activated Ras-Erk1/2 pathway (Schlessinger, 2004, Yu et al., 2001). However, c-Met inhibitors did not affect Erk1/2 or Smad1 Ser206 phosphorylation in MC3T3-E1 cells (Fig. 3B). These data suggest that c-Met inhibitors can promote osteoblast differentiation via pathways independent of Erk1/2-Smad. |
| ln Vivo |
SYN1143 (10-100 mg/kg; p.o. for 22 d) suppresses the growth of RON-expressing tumors that are constitutively active and c-Met-dependent[1].
SYN1143 (20-50 μg; transferred into calvarial defects) promotes the growth of new bone in mouse calvarial bone defects of critical size[2]. Compound I/SYN1143 inhibits the growth of c-Met–dependent and constitutively active RON-expressing tumors. [1] Although cell line models that depend on c-Met activity have been identified, few, if any, equivalent models are known for RON. Expression of c-Met, RON, or even their coexpression does not necessarily confer dependence on these pathways for tumor growth. Our goal was to identify models that were dependent on c-Met or expressed a constitutively active form of RON to investigate the in vivo antitumor activity of compound I. [1] Compound I/SYN1143 was assessed in two c-Met–dependent xenograft models: U-87 MG and NIH3T3 TPR-Met. Both models are well documented for their dependence on c-Met activity. Moreover, they both lack RON expression (Fig. 4A), eliminating the possibility that tumor growth inhibition might be mediated by blocking RON activity. [1] Treatment of NIH3T3 TPR-Met tumor-bearing mice once daily with compound I/SYN1143 resulted in a dose-dependent inhibition of tumor growth compared with vehicle-treated control animals (Fig. 5A). Compound I significantly inhibited tumor growth at doses of 30 or 100 mg/kg once daily or at 30 mg/kg twice daily (P < 0.02). Furthermore, complete tumor growth inhibition was observed at a dose of 100 mg/kg once daily. Treatment with compound I did not adversely affect body weight (data not shown). To confirm that tumor growth inhibition occurred as a result of inhibition of c-Met activity, mice bearing established NIH3T3 TPR-Met tumors were treated with a single dose of compound I at 100 mg/kg. Tumors were harvested from the mice at different times after dose and analyzed for TPR-Met and Gab1 phosphorylation. Phosphorylation levels of TPR-Met and Gab1 were profoundly reduced through 12 h and returned to near basal levels by 24 h when compared with tumors from untreated mice (Fig. 5B). Plasma levels of compound I in mice from experiments shown in Fig. 5A and B were evaluated. In both studies, mice treated with 100 mg/kg had high concentrations of compound I through 12 h but significantly lower concentrations at 24 h (Fig. 5C; data not shown). These data show that phospho-Gab1 and phospho-Met inhibition correlates with plasma levels of compound I, and in turn, these variables also correlate with NIH3T3 TPR-Met tumor growth inhibition. Compound I had no direct effect on Src phosphorylation in vivo despite the high concentration achieved in mice (Supplementary Fig. S2B). [1] To extend the finding that compound I/SYN1143 inhibits the growth of c-Met–dependent tumor models, compound I was tested against established U-87 MG tumor xenografts. As shown in Fig. 5D, compound I inhibited the growth of these tumors in a dose-dependent manner. Similar to the data in the NIH3T3 TPR-Met model, compound I significantly inhibited tumor growth at doses of 100 mg/kg once daily or 30 mg/kg twice daily (P < 0.0001). [1] We next tested the contribution of RONΔ160 in HT-29 tumor growth. When mice bearing established HT-29 tumors were dosed daily with compound I/SYN1143, a statistically significant, dose-dependent reduction in tumor growth was observed (P < 0.02; Fig. 6A). Mice bearing established HT-29 xenografts were also treated with a single dose of compound I at 100 mg/kg to assess the effect on ERK1/2 phosphorylation in vivo. ERK1/2 phosphorylation in the HT-29 xenografts was partially inhibited between 1 and 12 h following a single dose of compound I (Fig. 6B). The partial inhibition of ERK1/2 phosphorylation in vivo correlated with the partial inhibition of tumor growth (Fig. 6A) and with the partial inhibition of ERK1/2 phosphorylation observed in vitro (Fig. 4C). Interestingly, O'Toole and colleagues had similar findings in HT-29 xenografts using a monoclonal antibody to human RON. Together, these data suggest that RONΔ160 contributes to the oncogenic properties of HT-29 cells. [1] To confirm that compound I/SYN1143 does not have a nonspecific antitumor effect, we tested it in Colo205 xenografts that express both wild-type c-Met and RON (Fig. 4A) but show no signs of constitutive activity from either receptor (data not shown). Moreover, these cells, like HT-29 cells, express the activated b-Raf mutant V600E (38). Figure 6C shows that the growth of Colo205 tumors was unaffected by compound I treatment at doses that significantly inhibited tumor growth in c-Met–dependent models and in a model expressing RONΔ160. The c-Met chemical inhibitors stimulate bone formation in critical-sized defects of mouse calvarial bone [2] Damaged bone can be regenerated through osteoblastogenesis. The effect of c-Met inhibitor on bone regeneration was examined in a critical-sized calvarial defect of mouse. SYN1143 (20, 50 μg) or SGX523 (20, 50 μg) was transferred into calvarial defects with absorbable collagen sponges. After 3 weeks of SYN1143 or SGX523 treatment, bone regeneration regions were evaluated by μ-CT and histology. The two c-Met inhibitors produced marginal healing compared to the control group (Fig. 4A). Volumetric analysis of μ-CT showed that c-Met inhibitors induced bone formation in a dose-dependent manner compared to collagen sponge control (Fig. 4B, left panel). In area analysis for new bone formation within defects, SYN1143 (50 μg) and SGX523 (50 μg) produced new bones in 50% of the bony defect area. In the control group, only 10% of the area of bony defects was filled with new bones (Fig. 4B, right panel). Histology analysis showed that the defect regions with c-Met inhibitors delivery were filled with newly formed bone. In the control group, the defect regions were only filled with fibrous tissue without bone tissue (Fig. 4A). |
| Enzyme Assay |
Kinase assays. [1]
IC50 measurements of SYN1143/compound I for c-Met were measured by homogeneous time-resolved fluorescence as previously described with minor modification. A modified kinase reaction buffer was used that included 60 mmol/L HEPES (pH 7.4), 50 mmol/L NaCl, 20 mmol/L MgCl2, and 5 mmol/L MnCl2. Compound I was tested in a 10-point serial dilution using an ATP concentration of two-third Km value that was determined for c-Met and calculated using the Eadie-Hofstee and Lineweaver-Burke methods. The fluorescence ratios were read on a RubyStar instrument. To measure the IC50 of compound I for RON, the Invitrogen LanthaScreen procedure was followed and the fluorescein-poly-GT substrate was used. The fluorescence ratios were read on a Tecan Safire II. |
| Cell Assay |
Western blot analysis. [1]
Cells were grown to confluence, serum starved overnight, and then treated with various concentrations of SYN1143/compound I for 1 h. To investigate the ability of compound I to inhibit ligand-dependent receptor activation, serum-starved cells were treated with increasing concentration of compound I for 1 h and then stimulated with recombinant human HGF for 10 min (250 ng/mL) or MSP for 30 min (800 ng/mL) in the presence of compound I. These respective concentrations of growth factor were chosen based on previous dose-response studies indicating that these concentrations maximally activated their receptor. Immunoprecipitation. [1] Phosphorylated RON and RONΔ160 were immunoprecipitated from NIH3T3 RON cells and HT-29 cells, respectively, from 1 mg of cellular protein per sample using anti-phosphotyrosine 4G10 antibody. RON/RONΔ160 was subsequently detected with the RON C-20 antibody by Western blotting. Monolayer scratch assay. [1] NIH3T3 RON and NIH3T3 TPR-Met cells were seeded in six-well plates and grown until confluent. Cells were serum starved overnight and then treated with various concentrations of SYN1143/compound I for 1 h. A gap was introduced with a P200 pipette tip. In the case of NIH3T3 RON cells, cells were stimulated to migrate across the gap with 160 ng/mL of MSP C672A alone or MSP C672A and compound I, whereas NIH3T3 TPR-Met cells were treated with compound I only. After overnight incubation, the cells were examined by light microscopy and photographed. Magnifications (×40) are shown. Cell proliferation assay [1] Proliferation of cells in monolayer culture was analyzed using WST method with cell counting kit-8. Cells were cultured in 96-well plates in α-MEM or DMEM containing 10% FBS. Once achieving 70% confluence, medium was changed to α-MEM or DMEM containing 2.5% FBS without or with SYN1143 or SGX523 (0.3, 1, 3, 5, 10, 30 μM) and cultured for 24 h. CCK-8 solution (10 µl) was then added to each well and incubated at 37 °C for 1 h. Absorbance at 450 nm was determined using a microplate reader. α-MEM or DMEM containing 10% CCK-8 was used as control. |
| Animal Protocol |
Female CD1 nu/nu mice (6-8 weeks) bearing NIH3T3 TPR-Met s.c. tumors[1]
10, 30, 100 mg/kg Oral gavage either once or twice daily for 22 days Xenograft studies. [1] NIH3T3 TPR-Met (1 × 106), U-87 MG (5 × 106), HT-29 (2 × 106 with Matrigel at a ratio of 2:1), or Colo205 (2 × 106 with Matrigel) cells were injected s.c. in the right flank of female CD1 nu/nu mice (n = 10 per group). SYN1143/Compound I treatment began either 1 d after tumor implantation or when tumors were established (∼200 mm3). Compound I/SYN1143 was formulated in 2% hydroxypropylmethylcellulose 1% Tween 80 in water (pH 2.2 adjusted with HCl). Mice were dosed by oral gavage either once or twice daily. Tumor volume was measured twice weekly with a Pro-Max Fowler Digital Ultra Caliper (Fred Fowler Co., Inc.) as length (mm) × width (mm) × height (mm) and expressed as cubic millimeters. Data are expressed as mean ± SE for each group and plotted as a function of time. The statistical significance of observed differences between growth curves was evaluated by repeated measures ANOVA followed by Scheffe post hoc test. To assess the pharmacodynamic effect in tumors, mice bearing established (∼300–400 mm3) NIH3T3 TPR-Met or HT-29 xenograft tumors were treated with a single dose of compound I at 100 mg/kg. Tumors were harvested (n = 3 per time point) at the indicated times after treatment and immediately frozen in liquid nitrogen. Western blot analysis was performed to determine the effect of treatment on the phosphorylation of c-Met and Gab1, an adaptor protein immediately downstream of c-Met, or ERK1/2, which is downstream of RON. Animal studies, radiographic and histology analyses [2] Six-week-old male mice were randomly assigned to each experimental group. These animals were anesthetized with an intraperitoneal injection of a mixture of Zoletil (30 mg/kg) and Rompun (10 mg/kg. A critical-sized calvarial defect was created by using a 5-mm diameter trephine bur. The c-Met inhibitor SYN1143 or SGX523 was administered into the defect with absorbable collagen sponges. Three weeks after the administration, bone formation was evaluated by using a two-dimensional radiographic apparatus and three-dimensional micro-computed tomography (μ-CT) system. For soft X-ray analysis, scanned images were collected using diagnostic X-ray film under the following conditions: 35 kVp and 400 μA for 45 s. For μ-CT analysis, the scanned images were collected at 50 kV and 200 μA. These images were reconstructed using NRecon and CT analyzer software. 3D surface rendering image and repaired bone volume were obtained by using Mimics imaging program. For histology study, the implant or calvarial specimens were harvested, fixed in 10% neutral-buffered formalin, decalcified in Calci-Clear Rapid, embedded in paraffin, and then sectioned (4 µm in thickness). These sections were stained with hematoxylin/eosin to evaluate general tissue response and bone formation. |
| ADME/Pharmacokinetics |
Pharmacokinetics. [1]
Mouse plasma samples (20 μL) were extracted by using protein precipitation to isolate SYN1143/compound I and internal standard. Extracted samples were separated by reversed-phase liquid chromatography on a Varian Pursuit C18 analytic column (30 × 2.0 mm, 5 μm). The reagents were water with 0.1% formic acid (mobile phase A) and acetonitrile with 0.1% formic acid (mobile phase B). Gradient at a flow rate of 0.6 mL/min was used, with a total run time of 2 min. SYN1143/Compound I concentrations were determined in mouse samples by liquid chromatography-tandem mass spectrometry using ion atmospheric pressure ionization with multiple reaction monitoring in the positive ion mode. Peak areas were integrated by the Sciex program Analyst, version 1.4.1. The data were then exported to the software package Small Molecules Discovery Assay Watson where concentrations were determined by a weighted (1/×2) linear regression of peak area ratios (peak area of compound I/peak area of internal standard) versus the theoretical concentrations of the calibration standards. Overall precision and accuracy for the calibration standards and QC samples were determined by Small Molecules Discovery Assay Watson. |
| References |
|
| Additional Infomation |
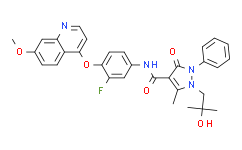
N-[3-fluoro-4-[(7-methoxy-4-quinolinyl)oxy]phenyl]-1-(2-hydroxy-2-methylpropyl)-5-methyl-3-oxo-2-phenyl-4-pyrazolecarboxamide is an aromatic amide.
Recepteur d'origine nantais (RON) is a receptor tyrosine kinase closely related to c-Met. Both receptors are involved in cell proliferation, migration, and invasion, and there is evidence that both are deregulated in cancer. Receptor overexpression has been most frequently described, but other mechanisms can lead to the oncogenic activation of RON and c-Met. They include activating mutations or gene amplification for c-Met and constitutively active splicing variants for RON. We identified a novel inhibitor of RON and c-Met, compound I, and characterized its in vitro and in vivo activities. Compound I selectively and potently inhibited the kinase activity of RON and c-Met with IC(50)s of 9 and 4 nmol/L, respectively. Compound I inhibited hepatocyte growth factor-mediated and macrophage-stimulating protein-mediated signaling and cell migration in a dose-dependent manner. Compound I was tested in vivo in xenograft models that either were dependent on c-Met or expressed a constitutively active form of RON (RONDelta160 in HT-29). Compound I caused complete tumor growth inhibition in NIH3T3 TPR-Met and U-87 MG xenografts but showed only partial inhibition in HT-29 xenografts. The effect of compound I in HT-29 xenografts is consistent with the expression of the activating b-Raf V600E mutation, which activates the mitogen-activated protein kinase pathway downstream of RON. Importantly, tumor growth inhibition correlated with the inhibition of c-Met-dependent and RON-dependent signaling in tumors. Taken together, our results suggest that a small-molecule dual inhibitor of RON/c-Met has the potential to inhibit tumor growth and could therefore be useful for the treatment of patients with cancers where RON and/or c-Met are activated. [1] The c-Met receptor tyrosine kinase and its ligand, hepatocyte growth factor (HGF), have been recently introduced to negatively regulate bone morphogenetic protein (BMP)-induced osteogenesis. However, the effect of chemical inhibitors of c-Met receptor on osteoblast differentiation process has not been examined, especially the applicability of c-Met chemical inhibitors on in vivo bone regeneration. In this study, we demonstrated that chemical inhibitors of c-Met receptor tyrosine kinase, SYN1143 and SGX523, could potentiate the differentiation of precursor cells to osteoblasts and stimulate regeneration in calvarial bone defects of mice. Treatment with SYN1143 or SGX523 inhibited HGF-induced c-Met phosphorylation in MC3T3-E1 and C3H10T1/2 cells. Cell proliferation of MC3T3-E1 or C3H10T1/2 was not significantly affected by the concentrations of these inhibitors. Co-treatment with chemical inhibitor of c-Met and osteogenic inducing media enhanced osteoblast-specific genes expression and calcium nodule formation accompanied by increased Runx2 expression via c-Met receptor-dependent but Erk-Smad signaling independent pathway. Notably, the administration of these c-Met inhibitors significantly repaired critical-sized calvarial bone defects. Collectively, our results suggest that chemical inhibitors of c-Met receptor tyrosine kinase might be used as novel therapeutics to induce bone regeneration. [2] |
| Molecular Formula |
C31H29FN4O5
|
|---|---|
| Molecular Weight |
556.58
|
| Exact Mass |
556.212
|
| Elemental Analysis |
C, 68.88; H, 5.41; F, 3.51; N, 10.36; O, 11.84
|
| CAS # |
913376-84-8
|
| Related CAS # |
913376-84-8
|
| PubChem CID |
16757524
|
| Appearance |
Light yellow to yellow solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Index of Refraction |
1.660
|
| LogP |
4.39
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
8
|
| Rotatable Bond Count |
8
|
| Heavy Atom Count |
41
|
| Complexity |
986
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=C(C1=C(C)N(CC(C)(C)O)N(C2C=CC=CC=2)C1=O)NC1C=C(F)C(OC2C3C(=CC(=CC=3)OC)N=CC=2)=CC=1
|
| InChi Key |
UYMSIPINLJNNOU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C31H29FN4O5/c1-19-28(30(38)36(21-8-6-5-7-9-21)35(19)18-31(2,3)39)29(37)34-20-10-13-27(24(32)16-20)41-26-14-15-33-25-17-22(40-4)11-12-23(25)26/h5-17,39H,18H2,1-4H3,(H,34,37)
|
| Chemical Name |
N-[3-fluoro-4-(7-methoxyquinolin-4-yl)oxyphenyl]-1-(2-hydroxy-2-methylpropyl)-5-methyl-3-oxo-2-phenylpyrazole-4-carboxamide
|
| Synonyms |
RON-IN-1; RONIN1; RON inhibitor 1; 913376-84-8; N-[3-Fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl]-1-(2-hydroxy-2-methylpropyl)-5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxamide; N-(3-Fluoro-4-((7-methoxyquinolin-4-yl)oxy)phenyl)-1-(2-hydroxy-2-methylpropyl)-5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxamide; N-{3-fluoro-4-[(7-methoxyquinolin-4-yl)oxy]phenyl}-1-(2-hydroxy-2-methylpropyl)-5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxamide; AMG-1; SYN1143; RON-IN-1; N-[3-fluoro-4-(7-methoxyquinolin-4-yl)oxyphenyl]-1-(2-hydroxy-2-methylpropyl)-5-methyl-3-oxo-2-phenylpyrazole-4-carboxamide; RON IN 1; Met Kinase Inhbitor IV ; RON inhibitor-1; Ron Inhibitor I
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: ~100 mg/mL (179.7 mM)
Ethanol: ~60 mg/mL (107.8 mM) |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (4.49 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.49 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (4.49 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7967 mL | 8.9834 mL | 17.9669 mL | |
| 5 mM | 0.3593 mL | 1.7967 mL | 3.5934 mL | |
| 10 mM | 0.1797 mL | 0.8983 mL | 1.7967 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.