| Size | Price | Stock | Qty |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| Targets |
TSPO/mitochondrial translocator protein
|
|---|---|
| ln Vitro |
In SHSY-5Y neuroblastoma cells, 4'-chlorodiazepam has neuroprotective properties against amyloid-β (Aβ). In organotypic hippocampal preparations, Aβ decreases cell viability but 4'-chlordiazepam, at doses of 100nM and 1000nM, is neuroprotective. The upregulation of superoxide dismutase (SOD) expression is associated with the neuroprotective effects of 4'-chlordiazepam on Aβ [1]. In glucose-deprived cells, 4'-clozazepam decreases nuclear fragmentation and maintains cell viability. In cells treated with 4'-chlordiazepam, these effects are accompanied by a decrease in the formation of free radicals and the preservation of mitochondrial function [2].
|
| Cell Assay |
The translocator protein (TSPO) is an outer mitochondrial membrane protein involved in the transport of cholesterol into the mitochondria, which is the first step for the synthesis of steroid hormones, as well as in the regulation of mitochondrial permeability transition pore opening and apoptosis. Studies have shown that the activation of TSPO may promote neuroprotective actions in experimental models of neurodegeneration and brain injury. In a previous study, our group showed that 4'-chlorodiazepam (4'-CD), a TSPO ligand, was neuroprotective against amyloid-beta (Aβ) in SHSY-5Y neuroblastoma cells. The aim of this study was to evaluate if 4'-CD was also neuroprotective against Aβ in organotypic hippocampal cultures and to identify its mechanisms of action. Aβ decreased the cell viability of organotypic hippocampal cultures, while 4'-CD had a neuroprotective effect when administered at 100nM and 1000nM. The neuroprotective effects of 4'-CD against Aβ were associated with an increased expression of superoxide dismutase (SOD). No differences were found in the expression of catalase, glial fibrillary acidic protein, Akt and procaspase-3. In summary, our results show that 4'-CD is neuroprotective against Aβ by a mechanism involving the modulation of SOD protein expression[1].
The translocator protein (TSPO), formerly known as the peripheral-type benzodiazepine receptor (PBR), is considered an important regulator of steroidogenesis and a potential therapeutic target in neurological disorders. Previous evidence suggests that TSPO ligands can protect cells during injury and prevent apoptosis in central nervous system (CNS) cells. However, its actions on astrocytic cells under metabolic injury are not well understood. In this study, we explored whether 4'-chlorodiazepam (Ro5-4864), a TSPO ligand, might protect astrocyte mitochondria under glucose deprivation. Our results showed that 4'-chlorodiazepam preserved cell viability and reduced nuclear fragmentation in glucose-deprived cells. These effects were accompanied by a reduced production of free radicals and maintenance of mitochondrial functions in cells treated with 4'-chlorodiazepam. Finally, our findings suggest that TSPO might be involved in reducing oxidative stress by preserving mitochondrial functions in astrocytic cells exposed to glucose withdrawal[2]. |
| References |
[1]. B D Arbo, et al. 4'-Chlorodiazepam is neuroprotective against amyloid-beta in organotypic hippocampal cultures. J Steroid Biochem Mol Biol. 2017 Jul;171:281-287.
[2]. Eliana Baez, et al. 4'-Chlorodiazepam Protects Mitochondria in T98G Astrocyte Cell Line from Glucose Deprivation. Neurotox Res. 2017 Aug;32(2):163-171. |
| Additional Infomation |
Mechanism of Action
Mitochondria isolated from rat brain were found to cleave cholesterol to produce pregnenolone, the precursor for hormonal steroids, at a mean rate of 21.0 pmol pregnenolone.mg protein-1.min-1. This rate-limiting step in steroidogenesis was significantly stimulated by PK 11195 (1-(2-chlorophenyl)-N-methyl-(1-methylpropyl)-3-isoquinoline carboxamide) and Ro5 4864 (4'-chlorodiazepam), ligands which bind to peripheral benzodiazepine receptors with high affinity. Low-affinity ligands for the peripheral benzodiazepine receptor such as Ro15 1788 (ethyl-8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5 alpha][1,4] benzo-3-carboxylate) and clonazepam had no significant effect on the rate of pregnenolone synthesis. Furthermore, the rank order of potency of these compounds as inhibitors of [3H]Ro5 4864 binding was identical to the rank order for steroid production. Since the 86-amino acid peptide diazepam binding inhibitor is also thought to bind to the peripheral benzodiazepine receptor, four fragments of this peptide, a random sequence and steroidogenesis activator peptide were also evaluated for their ability to interact with peripheral benzodiazepine receptors and to stimulate steroidogenesis in rat brain mitochondria. Steroidogenesis activator peptide and two fragments of diazepam binding inhibitor significantly stimulated pregnenolone biosynthesis. In contrast to the peripheral benzodiazepine receptor ligands, no correlation between peptide potency in displacing [3H]Ro5 4864 binding and steroidogenesis was observed. Peripheral benzodiazepine receptors mediate cholesterol translocation between the outer and inner mitochondrial membranes in steroidogenic tissues. They are found in many other tissues too, including liver. We studied the effect of the peripheral benzodiazepine receptor ligands PK11195 [1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoquinoline-3-carboxa mid e], Ro 5-4864 (4-chlorodiazepam), hemin, protoporphyrin IX and N-methyl protoporphyrin IX on cholesterol mitochondrial intermembrane transport of cholesterol in vitro in rat liver. Endogenous cholesterol translocation from outer to inner mitochondrial membranes was significantly increased by PK11195 and N-methyl protoporphyrin IX (140% and 150% increase, respectively, at 1 microM, P<0.01). 5 microM protoporphyrin IX, 1 microM Ro 5-4864 and 5 microM hemin was ineffective. When mitochondria were labeled with exogenous [4-14C]cholesterol, PK11195 and N-methyl protoporphyrin IX were the most effective in increasing total cholesterol incorporation and cholesterol translocation into inner membranes, and their effect was dose-dependent. These data suggest that in liver the binding to peripheral benzodiazepine receptors is related to cholesterol translocation and the interaction of ligands with these receptors may play a role in the complex mechanism of regulation of cholesterol traffic between liver mitochondrial membranes. Effects of various benzodiazepines were investigated in ovariectomized rat isolated uterus which had been chronically pre-treated with different female sex hormones: oestrogen, progesterone and oestrogen + progesterone. Uteri obtained from all groups developed a spontaneous, rhythmic activity. The spontaneous activity observed in control uterus was either inhibited in a concentration-dependent manner by diazepam, 4'-chlorodiazepam, clonazepam or 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxam ide (PK 11195), or was abolished in [Ca2+]o-free solution. Diazepam, 4'-chlorodiazepam, clonazepam and PK 11195 all caused a concentration-dependent relaxation of the [K+]o-pre-contracted uterus with the relative order of potency: PK 11195 > 4'-chlorodiazepam > diazepam > clonazepam. Administration of [Ca2+]o (1 microM to 10 mM) caused a concentration-dependent contraction of uterus, bathed in [Ca2+]o-free physiological salt solution obtained from different pre-treatment groups. Incubation with different concentrations (uM) of diazepam, 4'-chlorodiazepam, clonazepam and PK 11195 caused a decrease in response to [Ca2+]o-induced contraction in all groups of rat uteri. These results indicate that micromolar benzodiazepine binding sites exist in rat uterus. Diazepam, 4'-chlorodiazepam, clonazepam and PK 11195 caused relaxation of pre-contracted rat uterus and this effect may involve the inhibition of influx of [Ca2+]o and the relaxing effects of different benzodiazepines observed in this study can be modulated by pre-treatment with different female hormones. The interactions of the atypical benzodiazepine 4'-chlorodiazepam (Ro 5-4864) with functionally expressed human GABAA receptor cDNAs were determined. Cotransfection of human alpha 2, beta 1, and gamma 2 subunits was capable of reconstituting a 4'-chlorodiazepam recognition site as revealed by a dose-dependent potentiation of t-[35S]butylbicyclophosphorothionate ([35S]-TBPS) binding to the GABA-activated chloride channel. This site is found on GABAA receptor complexes containing sites for GABA agonist-like benzodiazepines and neuroactive steroids. The importance of the alpha subunit was further demonstrated as substitution of either alpha 1 or alpha 3 for the alpha 2 subunit did not reconstitute a 4'-chlorodiazepam recognition site that was capable of modulating [35S]TBPS binding under the same experimental conditions. The 4'-chlorodiazepam modulatory site was shown to be distinct from the benzodiazepine site, but the phenylquinolines PK 8165 and PK 9084 produced effects similar to 4'-chlorodiazepam, consistent with the previous analysis of the 4'-chlorodiazepam site in brain homogenates. Further analysis of the subunit requirements revealed that coexpression of alpha 2 and beta 1 alone reconstituted a 4'-chlorodiazepam recognition site. It is interesting, however, that the 4'-chlorodiazepam site was found to inhibit [35S]TBPS binding to the GABA-activated chloride channel. Thus, the 4'-chlorodiazepam site may be reconstituted with only the alpha and beta polypeptides. For more Mechanism of Action (Complete) data for 4-CHLORODIAZEPAM (6 total), please visit the HSDB record page. |
| Molecular Formula |
C16H12CL2N2O
|
|---|---|
| Molecular Weight |
319.185
|
| Exact Mass |
318.033
|
| Elemental Analysis |
C, 60.21; H, 3.79; Cl, 22.21; N, 8.78; O, 5.01
|
| CAS # |
14439-61-3
|
| PubChem CID |
1688
|
| Appearance |
Typically exists as white to light yellow solids at room temperature
|
| Density |
1.35g/cm3
|
| Boiling Point |
517.1ºC at 760mmHg
|
| Melting Point |
160-163ºC
|
| Flash Point |
266.6ºC
|
| Vapour Pressure |
8.43E-11mmHg at 25°C
|
| Index of Refraction |
1.647
|
| LogP |
3.307
|
| Hydrogen Bond Donor Count |
0
|
| Hydrogen Bond Acceptor Count |
2
|
| Rotatable Bond Count |
1
|
| Heavy Atom Count |
21
|
| Complexity |
432
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
ClC1C([H])=C([H])C2=C(C=1[H])C(C1C([H])=C([H])C(=C([H])C=1[H])Cl)=NC([H])([H])C(N2C([H])([H])[H])=O
|
| InChi Key |
PUMYFTJOWAJIKF-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H12Cl2N2O/c1-20-14-7-6-12(18)8-13(14)16(19-9-15(20)21)10-2-4-11(17)5-3-10/h2-8H,9H2,1H3
|
| Chemical Name |
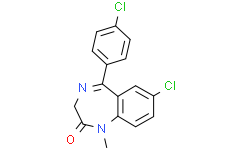
7-chloro-5-(4-chlorophenyl)-1-methyl-3H-1,4-benzodiazepin-2-one
|
| Synonyms |
RO-5-4864; RO 5-4864; Chlordiazepam; RO5-4864; 4-Chlorodiazepam; Ro 5-4864; 2H-1,4-Benzodiazepin-2-one, 7-chloro-5-(4-chlorophenyl)-1,3-dihydro-1-methyl-; Ro-05-4864; RO5-4864
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO : ~100 mg/mL (~313.29 mM)
|
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.83 mM) (saturation unknown) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 + to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1329 mL | 15.6647 mL | 31.3293 mL | |
| 5 mM | 0.6266 mL | 3.1329 mL | 6.2659 mL | |
| 10 mM | 0.3133 mL | 1.5665 mL | 3.1329 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.