
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
Purity: ≥98%
KU-60019 is an improved analogue of KU-55933 with 10-fold higher activity than KU-55933 at blocking radiation-induced phosphorylation of key ATM targets in human glioma cells. It is a very potent radiosensitizer with an IC50 of 6.3 nM for ATM in cell-free assays, and it is 270 and 1600 times more selective for ATM than DNA-PK and ATR. In human glioma cell lines that are p53 wild type (U87) and p53 mutant (U1242), KU-60019 can decrease radiation-induced S473 AKT phosphorylation. But AKT suppression does not improve KU-60019's radiosensitization. In human glioma cells U87 and U1242, KU-60019 has been shown to inhibit cell migration and invasion in a dose-dependent manner. Furthermore, it has been demonstrated that KU-60019 can inhibit the growth of U1242 cells to a certain degree.
| Targets |
ATM ( IC50 = 6.3 nM ); DNA-PKcs ( IC50 = 1.7 μM )
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
Radiation plus KU-60019 greatly increases mouse survival in orthotopic glioma U1242/luc-GFP xenograft models compared to radiation alone, KU-60019 alone, or no treatment at all. Furthermore, p53-mutant gliomas respond far more strongly to KU-60019 radiosensitization than do gliomas of the wild type.[2]
In vivo efficacy of ATM inhibition in PTEN-deficient xenografts To investigate the in vivo efficacy of ATM specific inhibition in PTEN-deficient cells, we used the PC3 PTEN Tetracycline inducible cell line model in a subcutaneous xenograft setting. Firstly, we established that the Tetracycline derivative Doxycycline was efficient at inducing PTEN expression in vitro (Supplementary Fig. 4A) and in vivo (Supplementary Fig. 4B). Calliper measurements of tumour volumes showed that induction of PTEN using Doxycycline, led to a slowing of tumour growth from 72 hours onwards (Supplementary Fig. 4C). Next, we investigated the selective toxicity of the ATM specific kinase inhibitor KU-60019 as a single modality in the PC3-PTEN inducible model. This inhibitor was chosen, as it is a potent ATM inhibitor and unlike KU-55933 is active in animal systems. Despite PTEN-deficient control tumours reaching a 4-fold increase in size before PTENwild-typecontrols, KU-60019 treated PTEN-deficient tumours displayed a statistically significant slowing in growth (Fig. 4A). This growth inhibition was especially evident at the start of the experiment (days 5-12) just after KU-60019 was administered (day 1-5). There were no significant changes in the mean relative body weights of each treatment groups (Fig. 4B). Inducible PTEN expression in vivo was analysed in resected tumours by immunofluorescence [2]. |
|
| Cell Assay |
Cell growth was determined by AlamarBlue®. U1242 cells were serially diluted, allowedto attach for 6 h and then exposed to KU-60019 at 3 μM. At days 1, 3 and 5 after seeding, AlamarBlue® was added to the medium to the recommended final concentration. Plates were incubated for 1 h at 37°C and fluorescence determined on a FluoroCount plate reader excitation 530 nm, emission 590 nm) and values taken as a measure of cell growth.[1]
KU-60019 is exposed to cells for 1, 3, and 5 days. AlamarBlue is the measure of cell growth. The medium is supplemented with AlamarBlue up to the suggested final concentration. The Fluoro-Count plate reader (excitation 530 nm, emission 590 nm) is used to measure fluorescence on the plates after an hour of incubation at 37 °C. The values obtained represent the growth of the cells. The trypan blue/fluorescence activated cell sorting (FACS) assay is used to assess cell survival. |
|
| Animal Protocol |
|
|
| References |
|
|
| Additional Infomation |
Ataxia telangiectasia (A-T) mutated (ATM) is critical for cell cycle checkpoints and DNA repair. Thus, specific small molecule inhibitors targeting ATM could perhaps be developed into efficient radiosensitizers. Recently, a specific inhibitor of the ATM kinase, KU-55933, was shown to radiosensitize human cancer cells. Herein, we report on an improved analogue of KU-55933 (KU-60019) with K(i) and IC(50) values half of those of KU-55933. KU-60019 is 10-fold more effective than KU-55933 at blocking radiation-induced phosphorylation of key ATM targets in human glioma cells. As expected, KU-60019 is a highly effective radiosensitizer of human glioma cells. A-T fibroblasts were not radiosensitized by KU-60019, strongly suggesting that the ATM kinase is specifically targeted. Furthermore, KU-60019 reduced basal S473 AKT phosphorylation, suggesting that the ATM kinase might regulate a protein phosphatase acting on AKT. In line with this finding, the effect of KU-60019 on AKT phosphorylation was countered by low levels of okadaic acid, a phosphatase inhibitor, and A-T cells were impaired in S473 AKT phosphorylation in response to radiation and insulin and unresponsive to KU-60019. We also show that KU-60019 inhibits glioma cell migration and invasion in vitro, suggesting that glioma growth and motility might be controlled by ATM via AKT. Inhibitors of MEK and AKT did not further radiosensitize cells treated with KU-60019, supporting the idea that KU-60019 interferes with prosurvival signaling separate from its radiosensitizing properties. Altogether, KU-60019 inhibits the DNA damage response, reduces AKT phosphorylation and prosurvival signaling, inhibits migration and invasion, and effectively radiosensitizes human glioma cells.[1]
Ataxia telangiectasia mutated (ATM) is an important signaling molecule in the DNA damage response (DDR). ATM loss of function can produce a synthetic lethal phenotype in combination with tumor-associated mutations in FA/BRCA pathway components. In this study, we took an siRNA screening strategy to identify other tumor suppressors that, when inhibited, similarly sensitized cells to ATM inhibition. In this manner, we determined that PTEN and ATM were synthetically lethal when jointly inhibited. PTEN-deficient cells exhibited elevated levels of reactive oxygen species, increased endogenous DNA damage, and constitutive ATM activation. ATM inhibition caused catastrophic DNA damage, mitotic cell cycle arrest, and apoptosis specifically in PTEN-deficient cells in comparison with wild-type cells. Antioxidants abrogated the increase in DNA damage and ATM activation in PTEN-deficient cells, suggesting a requirement for oxidative DNA damage in the mechanism of cell death. Lastly, the ATM inhibitor KU-60019 was specifically toxic to PTEN mutant cancer cells in tumor xenografts and reversible by reintroduction of wild-type PTEN. Together, our results offer a mechanistic rationale for clinical evaluation of ATM inhibitors in PTEN-deficient tumors.[2] |
| Molecular Formula |
C30H33N3O5S
|
|
|---|---|---|
| Molecular Weight |
547.67
|
|
| Exact Mass |
547.214
|
|
| CAS # |
925701-49-1
|
|
| Related CAS # |
|
|
| PubChem CID |
15953870
|
|
| Appearance |
Light yellow to khaki solid powder
|
|
| Density |
1.3±0.1 g/cm3
|
|
| Boiling Point |
786.6±60.0 °C at 760 mmHg
|
|
| Flash Point |
429.5±32.9 °C
|
|
| Vapour Pressure |
0.0±2.7 mmHg at 25°C
|
|
| Index of Refraction |
1.645
|
|
| LogP |
5.9
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
8
|
|
| Rotatable Bond Count |
5
|
|
| Heavy Atom Count |
39
|
|
| Complexity |
972
|
|
| Defined Atom Stereocenter Count |
2
|
|
| SMILES |
C[C@@H]1CN(C[C@@H](O1)C)CC(=O)NC2=CC3=C(C=C2)SC4=C(C3)C=CC=C4C5=CC(=O)C=C(O5)N6CCOCC6
|
|
| InChi Key |
SCELLOWTHJGVIC-BGYRXZFFSA-N
|
|
| InChi Code |
InChI=1S/C30H33N3O5S/c1-19-16-32(17-20(2)37-19)18-28(35)31-23-6-7-27-22(13-23)12-21-4-3-5-25(30(21)39-27)26-14-24(34)15-29(38-26)33-8-10-36-11-9-33/h3-7,13-15,19-20H,8-12,16-18H2,1-2H3,(H,31,35)/t19-,20+
|
|
| Chemical Name |
2-[(2S,6R)-2,6-dimethylmorpholin-4-yl]-N-[5-(6-morpholin-4-yl-4-oxopyran-2-yl)-9H-thioxanthen-2-yl]acetamide
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
5%DMSO+ 40%PEG300+5%Tween 80+50%ddH2O: 5.0mg/ml (9.13mM) (Please use freshly prepared in vivo formulations for optimal results.)
|
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8259 mL | 9.1296 mL | 18.2592 mL | |
| 5 mM | 0.3652 mL | 1.8259 mL | 3.6518 mL | |
| 10 mM | 0.1826 mL | 0.9130 mL | 1.8259 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
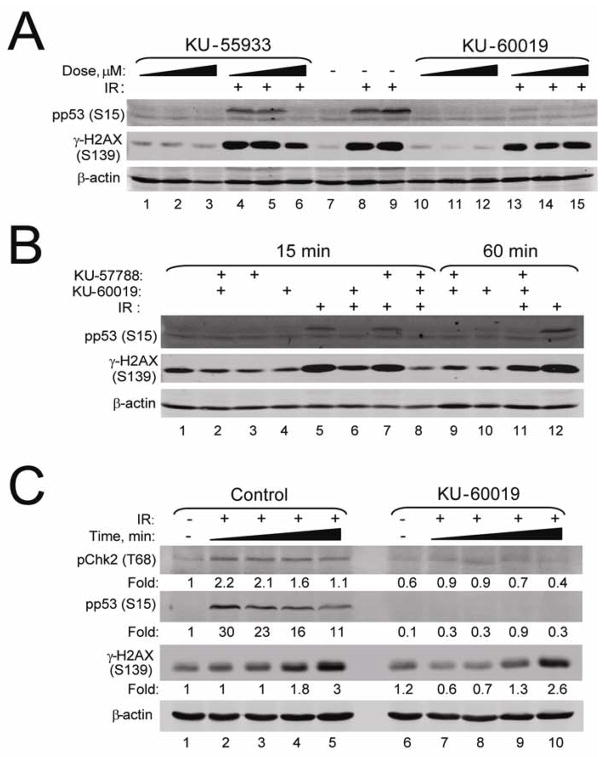 KU-60019 is a more effective inhibitor of the ATM kinase than KU-55933.Mol Cancer Ther.2009 Oct;8(10):2894-902. |
|---|
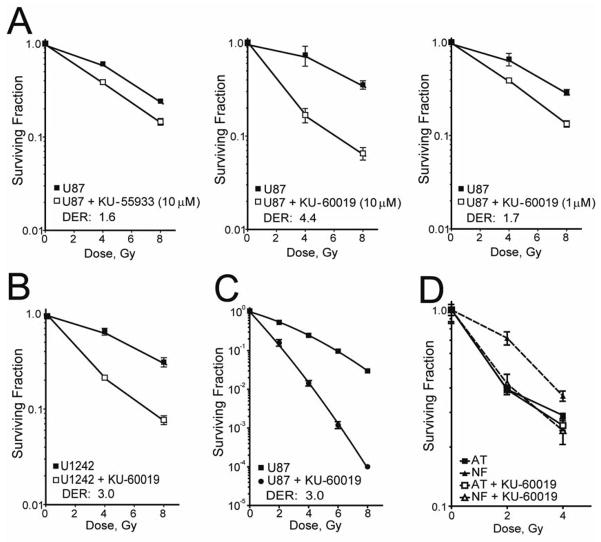 KU-60019 radiosensitizes U87 and U1242 human glioma cells and normal but not A-T fibroblasts.Mol Cancer Ther.2009 Oct;8(10):2894-902. |
KU-60019 inhibits migration and invasion of human glioma U87 and U1242 cells in vitro.Mol Cancer Ther.2009 Oct;8(10):2894-902. |