
| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| 5g | |||
| Other Sizes |
Purity: ≥98%
Cabozantinib malate (formerly known as XL-184 or BMS-907351; trade name Cometriq) is the malate salt form of Cabozantinib, a novel, potent and orally bioavailable VEGFR2 inhibitor with potential anticancer activity. Its IC50 of 0.035 nM is sufficient to inhibit VEGFR2. With IC50 values of 1.3 nM, 4 nM, 4.6 nM, 12 nM/11.3 nM/6 nM, 14.3 nM, and 7 nM in cell-free experiments, respectively, it is a multiple receptor tyrosine kinase inhibitor that also inhibits c-Met, Ret, Kit, Flt-1/3/4, Tie2, and AXL. Numerous RTKs, which are frequently overexpressed in a range of cancer cell types, are strongly bound to by cabotezantinib and inhibited.
| Targets |
VEGFR2/KDR (IC50 = 0.035 nM); c-Met (IC50 = 1.3 nM); RET (IC50 = 4 nM); Kit (IC50 = 4.6 nM); Flt-4 (IC50 = 6 nM)
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay |
As a strong inhibitor of VEGFR2, cabotinib (XL184, BMS-907351) has an IC50 of 0.035 nM. It also has IC50s of 1.3 nM, 4 nM, 4.6 nM, 12 nM/11.3 nM/6 nM, 14.3 nM, and 7 nM for c-Met, Ret, Kit, Flt-1/3/4, Tie2, and AXL, respectively.
|
|
| Cell Assay |
For 48 hours, cells are exposed to different dosages of cabotanzinib. Using the CellTiter96 Aqueous Non-Radioactive Cell Proliferation Assay kit, MTS assays are used to measure cell growth. The wavelength at which absorbance is measured is 490 nm, and the treated cells' absorbance values are expressed as a percentage of the untreated cells' absorbance.
|
|
| Animal Protocol |
RIP-Tag2 transgenic mice in a C57BL/6 background with spontaneous pancreatic islet tumors
~60 mg/kg Oral gavage |
|
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of cabozantinib during breastfeeding. Because cabozantinib is more than 97% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life ranges from 55 to 99 hours and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during cabozantinib therapy and for 4 months after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
|
| References |
|
|
| Additional Infomation |
Cabozantinib malate is a malate salt that is the mono-(S)-malate salt of cabozantinib. A multi-tyrosine kinase inhibitor, used for the treatment of progressive, metastatic, medullary thyroid cancer. It has a role as a tyrosine kinase inhibitor, an antineoplastic agent and a prodrug. It contains a cabozantinib.
Cabozantinib S-malate is the s-malate salt form of cabozantinib, an orally bioavailable, small molecule receptor tyrosine kinase (RTK) inhibitor with potential antineoplastic activity. Cabozantinib strongly binds to and inhibits several RTKs, which are often overexpressed in a variety of cancer cell types, including hepatocyte growth factor receptor (MET), RET (rearranged during transfection), vascular endothelial growth factor receptor types 1 (VEGFR-1), 2 (VEGFR-2), and 3 (VEGFR-3), mast/stem cell growth factor (KIT), FMS-like tyrosine kinase 3 (FLT-3), TIE-2 (TEK tyrosine kinase, endothelial), tropomyosin-related kinase B (TRKB) and AXL. This may result in an inhibition of both tumor growth and angiogenesis, and eventually lead to tumor regression. See also: Cabozantinib (has active moiety). Drug Indication Treatment of adult patients with progressive, unresectable locally advanced or metastatic medullary thyroid carcinoma. Renal Cell Carcinoma (RCC)Cabometyx is indicated as monotherapy for the treatment of advanced renal cell carcinoma (RCC): in treatment-naïve adults with intermediate or poor risk,in adults following prior vascular endothelial growth factor (VEGF)-targeted therapy. Cabometyx, in combination with nivolumab, is indicated for the first-line treatment of advanced renal cell carcinoma in adults. Hepatocellular Carcinoma (HCC)Cabometyx is indicated as monotherapy for the treatment of hepatocellular carcinoma (HCC) in adults who have previously been treated with sorafenib. |
| Molecular Formula |
C32H30FN3O10
|
|---|---|
| Molecular Weight |
635.59
|
| Exact Mass |
635.191
|
| Elemental Analysis |
C, 60.47; H, 4.76; F, 2.99; N, 6.61; O, 25.17
|
| CAS # |
1140909-48-3
|
| Related CAS # |
Cabozantinib;849217-68-1
|
| PubChem CID |
25102846
|
| Appearance |
White to off-white solid powder
|
| LogP |
4.593
|
| Hydrogen Bond Donor Count |
5
|
| Hydrogen Bond Acceptor Count |
12
|
| Rotatable Bond Count |
11
|
| Heavy Atom Count |
46
|
| Complexity |
924
|
| Defined Atom Stereocenter Count |
1
|
| SMILES |
O=C([C@H](CC(O)=O)O)O.O=C(NC1=CC=C(C=C1)OC2=CC=NC3=CC(OC)=C(C=C23)OC)C4(CC4)C(NC5=CC=C(C=C5)F)=O
|
| InChi Key |
HFCFMRYTXDINDK-WNQIDUERSA-N
|
| InChi Code |
InChI=1S/C28H24FN3O5.C4H6O5/c1-35-24-15-21-22(16-25(24)36-2)30-14-11-23(21)37-20-9-7-19(8-10-20)32-27(34)28(12-13-28)26(33)31-18-5-3-17(29)4-6-18;5-2(4(8)9)1-3(6)7/h3-11,14-16H,12-13H2,1-2H3,(H,31,33)(H,32,34);2,5H,1H2,(H,6,7)(H,8,9)/t;2-/m.0/s1
|
| Chemical Name |
1-N-[4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-1-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide;(2S)-2-hydroxybutanedioic acid
|
| Synonyms |
Cabozantinib malate; XL-184; BMS-907351; XL184; XL 184; BMS907351; BMS 907351; Cabozantinib S-malate. Brand name: Cometriq
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
|
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5733 mL | 7.8667 mL | 15.7334 mL | |
| 5 mM | 0.3147 mL | 1.5733 mL | 3.1467 mL | |
| 10 mM | 0.1573 mL | 0.7867 mL | 1.5733 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01709435 | Active Recruiting |
Drug: Cabozantinib S-malate Other: Pharmacological Study |
Recurrent Melanoma Recurrent Malignant Solid Neoplasm |
National Cancer Institute (NCI) |
November 14, 2012 | Phase 1 |
| NCT02867592 | Active Recruiting |
Drug: Cabozantinib S-malate Drug: Cabozantinib |
Ewing Sarcoma Hepatoblastoma |
National Cancer Institute (NCI) |
May 8, 2017 | Phase 2 |
| NCT02243605 | Active Recruiting |
Drug: Cabozantinib S-malate Other: Laboratory Biomarker Analysis |
Metastatic Ewing Sarcoma Metastatic Osteosarcoma |
National Cancer Institute (NCI) |
December 19, 2014 | Phase 2 |
| NCT02302833 | Active Recruiting |
Drug: Cabozantinib S-malate Other: Laboratory Biomarker Analysis |
Metastatic Paraganglioma Unresectable Paraganglioma |
M.D. Anderson Cancer Center | February 17, 2015 | Phase 2 |
| NCT01935934 | Active Recruiting |
Drug: Cabozantinib S-malate Other: Pharmacological Study |
Stage IV Uterine Corpus Cancer AJCC v7 Stage IVA Uterine Corpus Cancer AJCC v7 |
National Cancer Institute (NCI) |
April 29, 2013 | Phase 2 |
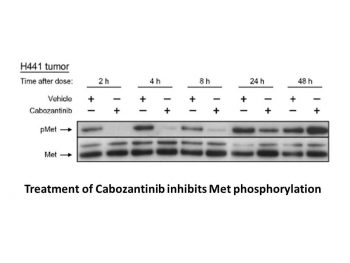 |
The multi-tyrosine kinase inhibitor, XL184, targeting MET and VEGFR2 abrogates MPNST migration, invasion, and angiogenesis. Clin Cancer Res. 2011 Jun 15;17(12):3943-55. |
XL184 abrogates local and metastatic MPNST growth in vivo. Clin Cancer Res. 2011 Jun 15;17(12):3943-55. |